
EndoSoft® announced the release of its one-of-a-kind data collection tool for IBD scoring. The IBD scoring scale was developed in collaboration and assistance from Dr. William J. Sandborn, the leading authority in IBD from UC San Diego where he is a board-certified gastroenterologist & professor of medicine. Dr. Sandborn directs a large clinical research unit devoted to the conduct of clinical trials in inflammatory bowel disease, where he supervises a multi-investigator team of physicians, research fellows, nurses, and study coordinators. With his physician collaborators, he develops and evaluates new diagnostic modalities and medical therapies for inflammatory bowel disease. He is internationally recognized for his contributions in the fields of biotechnology therapy, clinical pharmacology, conduct of clinical trials, diagnostic and treatment of pouchitis, epidemiology, natural history, and endoscopic & radiographic imaging techniques.
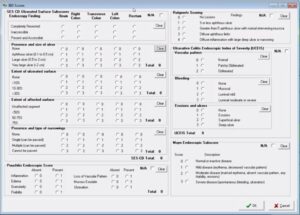
The IBD scoring scale is a comprehensive tool which helps collect data on all aspects necessary for a thorough record, such as ulcerated surface subscores, pouchitis endoscopic scores, Rutgeerts scoring, and Mayo endoscopic subscores.
EndoVault® stands out as a powerful tool for patient care and research due to its seamless data collection capabilities and its integrated, state-of-the-art data analytics tool. This combination allows for comprehensive data extraction, both collectively and for individual scoring scales.
About EndoSoft®
EndoSoft® is a leading healthcare software technology company offering best-of-breed software modules for endoscopy procedure documentation, image management, anesthesia documentation, pathology requisition and results, Electronic Nursing Records (ENR®), quality reporting system, scheduling, patient tracking, e-prescriptions and PACS. With over 25,000 clinical users worldwide, EndoSoft® has achieved a leadership position and a reputation for excellence and quality.
From hospitals and ASCs to office practices, EndoSoft® applications are designed to suit the specific needs of individual specialties including Gastroenterology, Oncology, Pulmonology, Pain Management, Urology, General Surgery, Cardiology, Orthopedics, Pathology, OB/GYN, Plastic Surgery, Podiatry, Ophthalmology and Dermatology. EndoSoft® applications allow medical professionals to integrate their patient data, administrative, business, clinical data and image management systems. Applications work seamlessly with each other and with all major enterprise-wide applications on the market, offering increased efficiency, enhanced quality of care and cost savings.
EndoSoft® Data collection tool has the ability to connect the data extraction from several sites using EndoSoft® applications to collect and evaluate data on a larger scale to contribute to IBD research.
EndoSoft® applications are fully prepared for the ICD-10 transition in October 2015. They are integrated and certified with GIQuIC and NED, enabling seamless reporting for CMS Quality Measures, ASC 9/10, OP 29/30, and other endoscopy metrics. These reporting features are built into the workflow to minimize redundant data entry.

